Cell rearrangement
Mechanism of cell junction exchange during cell rearrangement
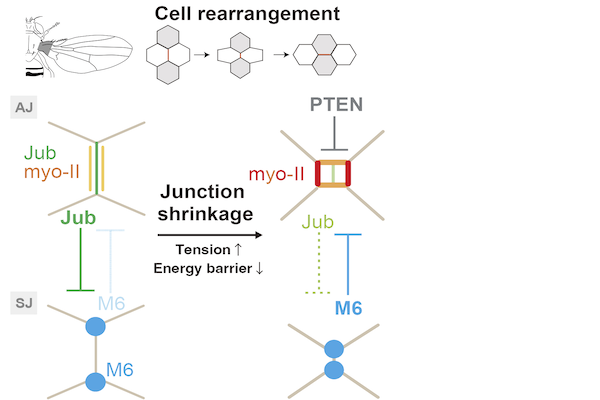
Cell rearrangement plays a fundamental role in shaping a tissue and developing multicellular patterns. Cell rearrangement proceeds in three steps: the shrinkage of a junction, exchange of junctions around the cell vertex, and elongation of the newly generated junction. Although the molecular mechanisms underlying junction shrinkage and elongation have been well characterized, little is known regarding how epithelial cells exchange junctions during cell rearrangement. Herein, by combining live imaging and physical modeling, we showed that the formation of myosin-II (myo-II) cables around the cell vertices underlies the exchange of junctions in the Drosophila wing epithelium. The local and transient detachment of myo-II from the cell cortex is regulated by the LIM domain-containing protein Jub and the tricellular septate junction protein M6. Moreover, we found that M6 shifts to the adherens junction plane upon jub RNAi and that Jub is persistently retained at reconnecting junctions in m6 RNAi cells. This interplay between Jub and M6 can depend on the junction length and thereby couples the detachment of cortical myo-II cables and the shrinkage/elongation of the junction during cell rearrangement. Furthermore, we developed a mechanical model based on the wetting theory and clarified how the physical properties of myo-II cables are integrated with the junction geometry to induce the transition between the attached and detached states and support the unidirectionality of cell rearrangement. Collectively, the present study elucidates the orchestration of geometry, mechanics and signaling for exchanging junctions.Mechano-sensation and mechano-resistance during cell rearrangement
The global patterns of forces in a tissue (e.g., tissue tension/compression) control many aspects of development including cell proliferation, cell rearrangement, and cell polarity. Such control relies on the ability of cells to sense the distribution of forces and tune morphogenetic signaling pathways in response to the mechanical inputs. Moreover, cells must resist or release tension/compression when deforming, proliferating, and moving during development. Whilst an understanding of molecular mechanisms for stress generation has evolved in the past decade, much less is known on how cells respond to and resist such stresses at the molecular level during morphogenesis. Here, we address the question in the Drosophila wing epithelium, where anisotropic tissue tension orients cell rearrangements. We found that anisotropic tissue tension localizes actin interacting protein 1 (AIP1), a cofactor of cofilin, on the remodeling junction via cooperative binding of cofilin to F-actin. AIP1 and cofilin promote actin turnover and locally regulate the Canoe-mediated linkage between actomyosin and the junction. This mechanism is essential for cells to resist the mechanical load imposed on the remodeling junction perpendicular to the direction of tissue stretching. Thus, the present study delineates how AIP1 and cofilin achieve an optimal balance between resistance to tissue tension and morphogenesis.References
AIP1 and cofilin ensure a resistance to tissue tension and promote directional cell rearrangement.
Nature Communications 9: 3295 (2018). [Journal] [Press release]
Attachment and detachment of cortical myosin regulates cell junction exchange during cell rearrangement in the Drosophila wing epithelium.
Current Biology 33: 263-275.E4 (2023) [Journal] [Press release (JP)] [Press release (EN)]